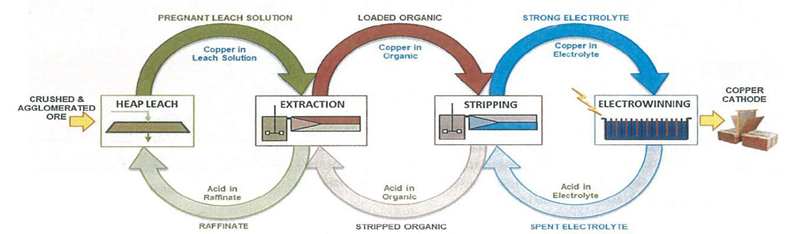
Processing
Heap Leach
A leach pad is defined as a continuously lined area which may contain one or more ‘heaps’.
Heaps are stacked on prepared pads to a height from 4 to 8 metres. The nominal heap height for Lady Annie Operations is 6 metres. Each heap is approximately 60 to 70 metres wide and may contain between 200,000 and 300,000 tonnes of ore. Heap leach solution is irrigated over the ore and percolates by gravity through the ore to be collected at the base of the pad in a collection drainage system. As the solution passes through the ore various metals including copper (Cu) are dissolved from the ore into the passing leach solution (leachate). The primary chemical in the leach solution is sulphuric acid (H2SO4), the copper (Cu) is solubilised.
The irrigated solution (raffinate) averages approximately 0.06 grams per litre of copper and 7 grams per litre of free acid. The solution discharging from the heaps, known as Pregnant Leach Solution (PLS), will vary in copper concentration over the life of the heap, initial grades may be as high as 10 grams per litre of copper. At the completion of leaching the PLS grade may be only fractionally greater than the raffinate irrigated.
When transitional ores are stacked air is forced into the heaps via a network of pipes located 1 metre above the base of each heap, the target minimum flow of air is one meter cubed of air per one meter squared of leach pad area per hour (1m3/hr/m2). The air provides oxygen to both the organic and inorganic reactions occurring within the heap. Within limitations, increasing the amount of air flow into a heap will increase the temperature within the heap. Conversely increasing the irrigation flow rate to the ore will reduce the temperature of the heap.
Solvent Extraction
The purpose of Solvent Extraction (SX) is to receive an impure solution (from the heap leach) containing dissolved copper and other metals, then remove (extract) that copper to transfer it to a synthetic and relatively pure solution from which the metal can be electrowon with minimal contamination by other metals.
Electrowinning
Electrowinning is the process of forming solid metal from a solution containing dissolved metal (electrolyte) by way of electrical deposition.
Direct Current (DC) is passed from anodes to cathodes depositing the copper at the surface of the cathode. The deposition process ordinarily requires 6 days at full production rate or longer at reduced rates before the cathodes are removed and stripped of the accumulated copper deposit (plate).
Lady Annie Operations use permanent stainless-steel cathode plates (Mother Plates) with synthetic 2-sided edge strips to carry the deposited copper plate. The anodes are composed of a Lead-Tin-Calcium alloy (Pb/Sn/Ca) with synthetic isolators to prevent short circuiting between the anodes and cathodes.
The Electrowinning plant consists of electrowinning cells each containing 33 cathodes and 34 anodes. The cathodes and anodes in each cell share common bus bars and operate in parallel electrically. The Cells are arranged in electrical series, each cell requires between 2.05VDC and 2.25VDC to drive the maximum electrical current of 24,000amps.
The Lady Annie Operations electrowinning plant has two distinct cell houses within series each being powered by one Transformer/Rectifier (Rectiformer) unit. The cell houses can be operated in isolation if required. Both cell houses share a common electrolyte system.
Cathode Stripping
Lady Annie Operations utilises a ‘Live Stripping’ technique to harvest the plated copper. Cathode plates are removed from the active cells in lots of 11 plates per extraction. The stripped cathode plates are returned to the cell before any other cathodes are removed to ensure a minimum of 22 plates remain in the cell at any given time to accommodate the current flow.
The stripping process handles one cathode plate at a time, the loaded plate is held in a frame vertically and flexed by air actuated rams to break the bond between the mother plate and copper cathode plate. A pair of separating tools (Knives) is driven from above to force the copper away from the stainless plate. Grippers are used to manoeuvre the copper plate into a horizontal position where it is collected to form bundles.
Each bundle will consist of approximately 42 copper plates at 60kg each for a total mass of 2,500Kg. One plate from every second bundle is sampled manually by a punch for quality verification. The bundles are then strapped and labelled ready for transport.